Hplc System Suitability
Definition Of System Suitability Test Limits On The Basis Of Robustness Test Results Omics International
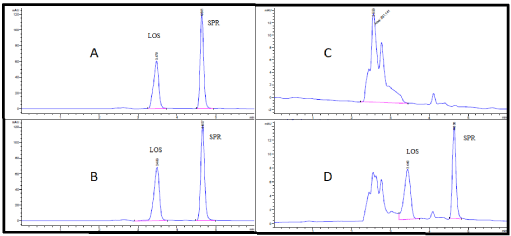
Developing A Highly Validated And Sensitive Hplc Method For Simultaneous Estimation Of Losartan And Spironolactone In Tablets And Human Plasma

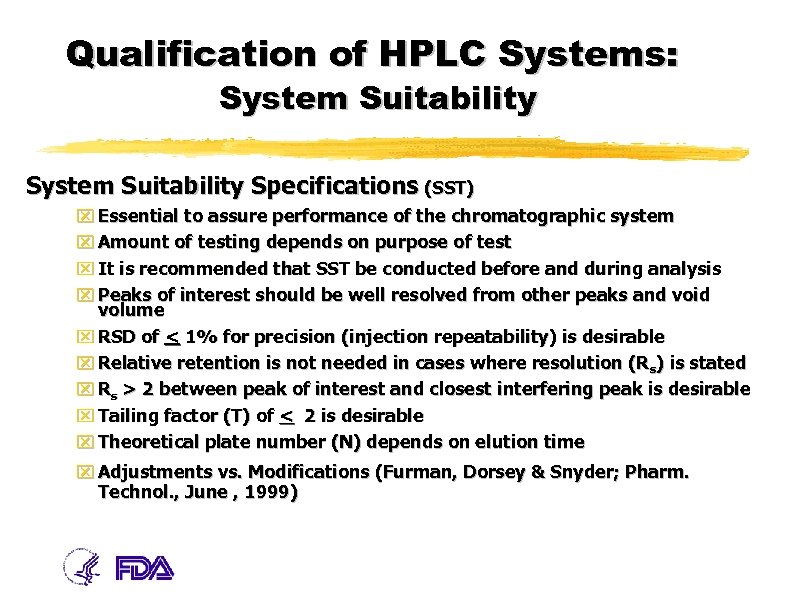
System Suitability Numerical Criteria For The Hplc Analysis

J System Suitability Specifications And Tests High Performance Liquid Chromatography Elution

Mourne Training Services System Suitability Failures

Hplc Calibration Process Parameters In Terms Of System Suitability Test Semantic Scholar
With the Arc HPLC System, the USP assay for losartan potassium met all of the system suitability requirements, including injection precision.
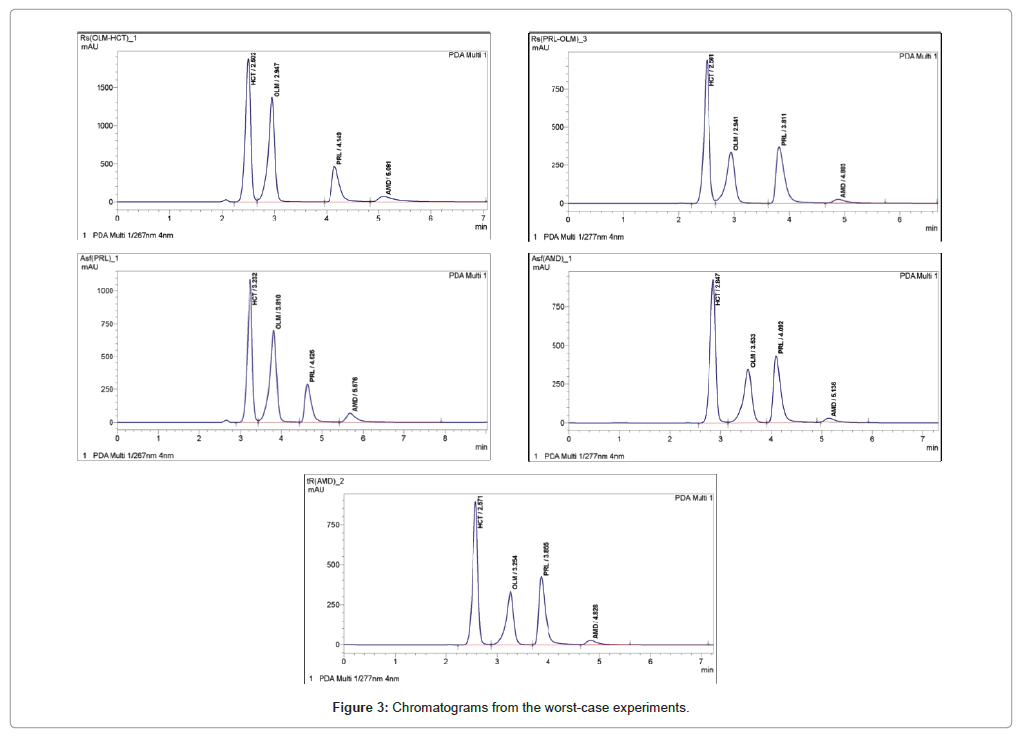
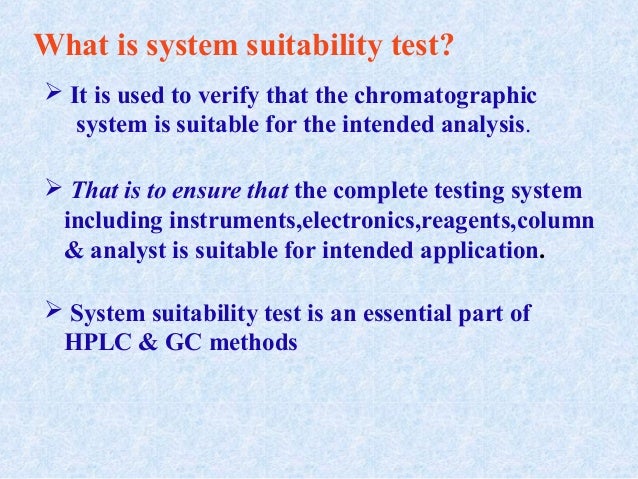
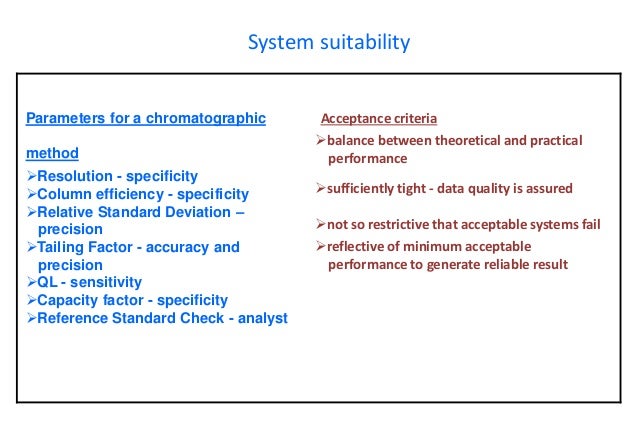
Hplc system suitability. System suitability The system suitability test ensures that the complete testing system, including instrument, reagents, column and analyst, is suitable for the intended application For that purpose, six consecutives injections were made with the standard solution of PAR, IBU, OLA, SIM and SIMA at a concentration of 75 µ mL −1. Evaluating System Suitability System Suitability Formulae and Calculations System Suitability Formulae and Calculations The HP ChemStation uses the following formulae to obtain the results for the various System Suitability tests The results are reported using the Performance, Performance Noise and Performance Extended report styles. Hplc system suitability All hplc system suitability wholesalers & hplc system suitability manufacturers come from members We doesn't provide hplc system suitability products or service, please contact them directly and verify their companies info carefully.
The System Suitability Testing (SST) is used to verify that an analytical method was suitable for its intended purpose the day the analysis was done It is an essential parameter to ensure the quality of the method for correct measurements. Always I thought that the SS validate the HPLC system before the sample's injections (first 5 or 6) but is true too that we can use the SS for all std injections but, is a risky decision to do that So, what do think about the fact that "that people" wants to eliminate one injection of the SS (considering the first 5) because of the RSD>%. Performance Qualification is usually performed after repair or regular system service procedures have been performed Using the same procedures for OQ and PQ simplifies the handling 213 System Suitability Check (SSC;.
HPLC • influence of pH in mobile phase • variations in mobile phase • different column • temperature • flow rate 9 System Suitability Testing Integral part of analytical procedures Title Microsoft Word 36 18 Stability Workshop ICH Q2B C doc Author. System Suitability In addition, prior to the start of laboratory studies to demonstrate method validity, some type of system suitability must be done to demonstrate that the analytical system is performing properly Examples include • replicate injections of a standard preparation for HPLC and GC methods;. System suitability shall be established as per the test procedure After every defined sample injections or after every test (Club analysis or individual analysis) standard preparation in single shall be injected (Called as Bracketing standard preparation).
• Enabling System Suitability • If you want system suitability to be calculated you will need to tell the processing Method to Calculate system suitability • Open the processing Method and select the Suitability Tab • Check the “Calculate Suitability Results” box • Complete the other sections as required ©05 Waters Corporation. During the routine analysis of drug and analytes System Suitability Test (SST) is one of the most important and integral parts of HPLC method development and calibration System Suitability Test (SST) is generally performed to evaluate the suitability and effectiveness of the entire chromatographic system not only prior to use but also during the time of analysis. System suitability Let us discuss first column compatibility with available HPLC system The key limitations are the detector flow cell volume and injection volume Pump parameters are usually bring no limitations unless you are planning to do either microcolumn separations or ultrafast analyses.
Many assay and impurity tests are now HPLC, and it is expected that the precision of these assays be equal or less than the RSD's for system suitability testing. System suitability testing limits are the acceptance criteria that must be met prior to the use of sample analysis The system suitability testing limit should conform to criteria provided in guidelines by CDER and other pharmacopeial references like USP and ICH Some of the parameters which can be checked as SST requirements are Capacity Factor. 52 Perform a system suitability test of all HPLC instruments prior to use and after completion of testing 53 The results of the different parameters which are included in system suitability should be within the limit as per the respective method of analysis.
The RSD of five standard injection and System suitability injection shall be NMT 2% During analysis, the flow rate of mobile phase shall be kept constant for the entire run after the system suitability is established If the flow rate of the system flows for more than 0 minutes, fresh system suitability shall be established. SYSTEM SUITABILITY System suitability tests are an integral part of gas and liquid chromatographic methods They are used to verify that the detection sensitivity, USP29 (Official June 1, 06) resolution, and reproducibility of the chromatographic system are adequate for the analysis to be done. System Suitability in HPLC Analysis System suitability is to prove that system is working perfectly before the analysis on HPLC, GC, TOC analyzer or any other system It is required to done before every sample analysis Ankur Choudhary Print Question Forum 3 comments.
System Suitability Test The third layer of the data quality triangle is the system suitability test Again the basis for a SST working reliably is that the instrument is qualified and the method used is validated USP defines this as “Verify that the system will perform in accordance with the criteria set forth in the procedure”. System Suitability Under the system suitability for purity (6) related substance in the Japanese Pharmacopoeia Sixteenth Edition, the "Test for required detection," "System performance," and "System reproducibility" are specified The specification values and the analysis results obtained are summarized below. Maintenance of the HPLC System If not correctly maintained the HPLC instrumentation can contribute to system suitability testing failures, eg worn injector parts may result in repeatability failure, an aged lamp in a UV detector can cause baseline noise and have a detrimental effect on the quantification of low level analytes.
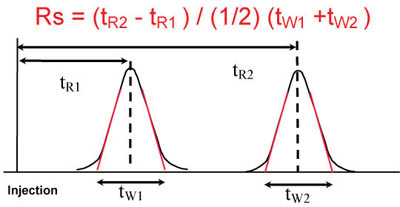
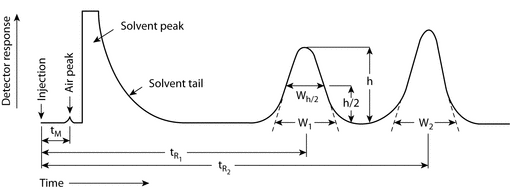
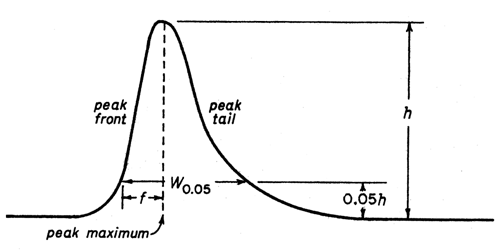
The following equations are related to System Suitability Please click on the corresponding tab below for the equations and details of how to calculate each one Calculation of the number of Theoretical Plates (halfheight method, used by Tosoh) Calculation of the number of Theoretical Plates (USP method). •2nd Review Method for Compliance –Is The Procedure Being Followed Properly?. How Do You Fix It?.
•1st Did System Suitability or Sample Fail?. HPLC Primaide An affordable and robust HPLC system engineered for seamless integration and reliable analyses The Hitachi HPLC Primaide is designed for longterm, stable operation, and features both high reliability and great durability It offers tremendous capability for everyday analysis. System suitability test เป็นกระบวนการตรวจสอบเพื่อให้มั่นใจว่า Chromatograph system (instrument, solvent, column, detector, pump, HPLC condition) เหมาะสมกับสารที่วิเคราะห์.
The system suitability test is used to verify that the chromatographic system is suitable for the intended analysis or not That is to ensure that the complete testing system including instruments, electronics, reagents, column & analyst is suitable for intended application The main system suitability parameters are 1 Precision 2 Capacity. How to calculate System Suitability in Chromatography HighPerformance Liquid Chromatography (HPLC) technique is applied in various places to separate out a mixture’s components Through this process, the liquid’s effectiveness is examined by passing it over an absorbent material. HPLC Primaide An affordable and robust HPLC system engineered for seamless integration and reliable analyses The Hitachi HPLC Primaide is designed for longterm, stable operation, and features both high reliability and great durability It offers tremendous capability for everyday analysis.
Tips and Tricks of HPLC System Troubleshooting Agilent Technologies, Inc LC Tips And Tricks Seminar Series Page 2 Trouble Shooting Steps You Have Recognized There is a Problem!. The system suitability test was developed for the routine application of the assay method, ensuring the adequacy of the proposed HPLC method The precision test and the tailing factor studies show good injection repeatability and peak symmetry, respectively. The system suitability test was developed for the routine application of the assay method, ensuring the adequacy of the proposed HPLC method The precision test and the tailing factor studies show good injection repeatability and peak symmetry, respectively ( Table 2 ).
Guest Author Dr Heiko Behr– European Pharmaceutical Senior Business Development Manager The European Pharmacopeia (EP) Chapter 2246 contains information that is similar to the USP Chapter 621 This includes general information about all chromatographic separations techniques, system suitability definitions and requirements, and chromatographic condition adjustments, also known as. System suitability testing (SST) is required by USP, FDA and EP to check and ensure ongoing performance of an analytical systems and methods Both USP and EP have chapters with recommendations for system suitability tests that are enforced by FDA and EMA Related chapters have been updated by USP and EP and they also answer the question as to. The system suitability is there to establish that your chromatography system is capable of performing analysis to the required standrad you require for successful analysis so I would say , yes.
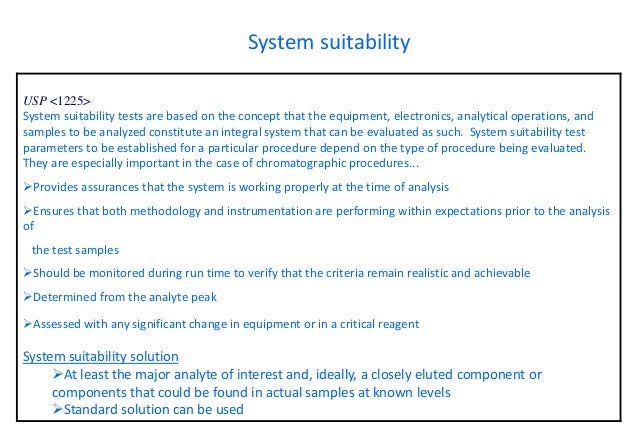
System suitability is widely recognized as a critical component of bioanalysis This paper discusses a generic system suitability test that monitors instrument performance throughout a run when used for liquid chromatography tandem mass spectrometry (LC/MS/MS) in bioanalysis. OBJECTIVE To lay down the procedure for operation of HPLC (Waters Alliance 2695 Separation Module) SCOPE To ensure that the instrument performs satisfactory and gives accurate and reproducible data This SOP shall be applicable for the HPLC system (Waters Alliances 2690 / 2695 Separation Modules RESPONSIBILITY Quality Control Officer/Executive. System suitability testing is an integral part of many analytical procedures Tests are based on the concept that the equipment, electronics, analytical operations and samples to be analyzed.
UV detectors are undoubtedly the most frequently used type of detector with HPLC systems, and I predict this will continue for many years, despite the rise of the modular mass spectrometric detector when the auxochrome is conjugated with a πelectron system, in order to evaluate analyte for its suitability for UV detection, its always. บันทึกเกี่ยวกับ HPLC (ปี 2557 2559) HPLC System suitability test # 2 HPLC System suitability test # 1 HPLC Change Active inlet valve HPLC Needle seat # 3 HPLC Needle seat # 2 HPLC Needle seat # 1 HPLC Signal option HPLC Size % of Page HPLC Steps Cleaning Plunger & Support Ring. Isolating HPLC Problems In an HPLC system, problems can arise from many sources First define the problem, then isolate the source Use Table 1 to determine which component(s) may be causing the trouble A process of elimination will usually enable you to pinpoint the specific cause and correct the problem.
System suitability Let us discuss first column compatibility with available HPLC system The key limitations are the detector flow cell volume and injection volume Pump parameters are usually bring no limitations unless you are planning to do either microcolumn separations or ultrafast analyses. System suitability involves measuring the accuracy, precision, linearity, specificity, sensitivity, and limits of detection for the method Detailed instructions for this stage can be found in the System Suitability Requirements Section Demonstrating system suitability generally requires about 40 injections and makes for a good independent. In accordance with the Rules and Procedures of the 05–10 Council of Experts, USP has postponed indefinitely the implementation requirement of text pertaining to System suitability, Detection sensitivity requirements published in the USP 29–NF 24, which becomes official on June 1, 06 On the basis of comments received, the General.
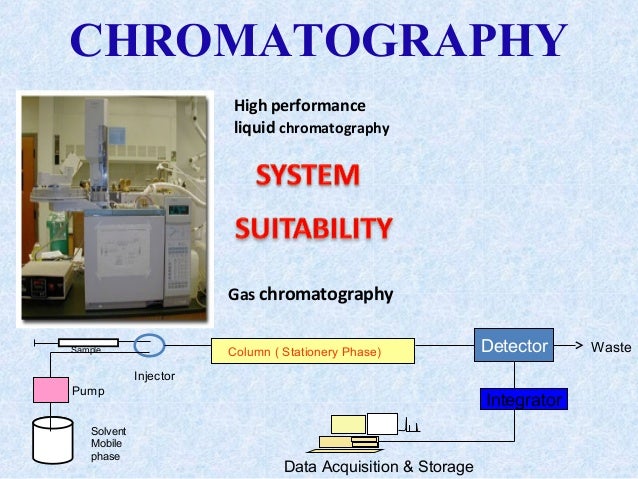
Highpressure liquid chromatography (HPLC), sometimes called highperformance liquid chromatography, is a separation technique based on a solid stationary phase and a liquid mobile phase Separations are achieved by partition, adsorption, or ionexchange processes, depending upon the type of stationary phase used System suitability tests. Also System Suitability Test, SST) The purpose of SSC is to prove and document that the necessary limits are met for a specific. For many analysts in the pharmaceutical analysis laboratory, the most familiar use of replicates in standard measurement is the 6 injections performed as part of system suitability testing for.
Chapter 1 describes the System Suitability software and its place in an HPLC system Chapter 2 describes how to install the System Suitability software and how to load the contents of the project included on the System Suitability disk Chapter 3 describes the equations that Empower software uses to determine system suitability Related. What is system suitability test?. HPLC Primaide An affordable and robust HPLC system engineered for seamless integration and reliable analyses The Hitachi HPLC Primaide is designed for longterm, stable operation, and features both high reliability and great durability It offers tremendous capability for everyday analysis.
System suitability The system suitability test ensures that the complete testing system, including instrument, reagents, column and analyst, is suitable for the intended application For that purpose, six consecutives injections were made with the standard solution of PAR, IBU, OLA, SIM and SIMA at a concentration of 75 µ mL −1. 3 SYSTEM SUITABILITY TESTS (SST) Once a method or system has been validated the task becomes one of routinely checking the suitability of the system to perform within the validated limits The simplest form of an HPLC system suitability test involves a comparison of the chromatogram trace with a standard trace (as shown below). Getting the peaks perfect System suitability for HPLC Online testing can ensure data quality in pharmaceutical assays Chromatography, specifically liquid chromatography, is used extensively in pharmaceutical development and manufacturing.
The system suitability (once established) shall be valid for a maximum period of 24 hours System suitability testing should be performed before the injection of samples No sample analysis is acceptable unless the requirements of system suitability have been met Sample analyses obtained while the system fails requirements are unacceptable. System suitability testing limits are the acceptance criteria that must be met prior to the use of sample analysis The system suitability testing limit should conform to criteria provided in guidelines by CDER and other pharmacopeial references like USP and ICH. Flush the HighPerformance Liquid Chromatography (HPLC) system with hot water (Approx Temp 5060 0 C or as per the suitability of tubing) by using union in place of Column at least by weekly Preparation of mobile phase and usage of solvent for Chromatography.
The following equations are related to System Suitability Please click on the corresponding tab below for the equations and details of how to calculate each one Calculation of the number of Theoretical Plates (halfheight method, used by Tosoh) Calculation of the number of Theoretical Plates (USP method).

Pharmapur Interview Questions On System Suitability Facebook
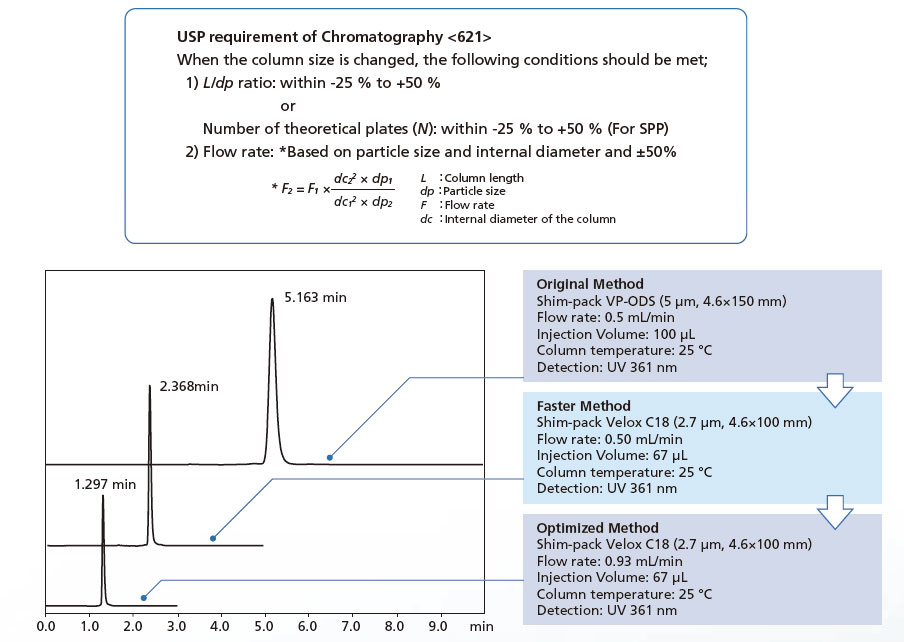
621 Chromatography

Analytical Parameters For System Suitability Test Of Hplc Method Download Table
Mohsinalsaleh Com Wp Content Uploads 17 03 12 Regulatory Aspects Of Hplc Analysis System Suitability 2 Pdf
Shim Pack Velox C18 Shimadzu Shimadzu Corporation

Hplc System Suitability Parameters For 5 Asa Analysis Download Scientific Diagram

Transfer Of Usp Assay For Quetiapine Fumarate Across Different Liquid Chromatographic Systems Waters

System Suitability Parameters Of Hplc Resolution Retention Time Tailing System Suitability Youtube

Hplc Calibration Process Parameters In Terms Of System Suitability Test Semantic Scholar
Definition Of System Suitability Test Limits On The Basis Of Robustness Test Results Omics International

Study And Ich Validation Of A Reverse Phase Liquid Chromatographic Method For The Quantification Of The Intact Monoclonal Antibody Cetuximab Sciencedirect
Www Mdpi Com 79 63 8 4 268 Pdf
System Suitability Parameters Of Hplc Resolution Retention Time Tailing System Suitability Youtube

System Suitability In Hplc Analysis Pharmaceutical Guidelines
Hplc

System Suitability Tests For Quantitative Chromatographic Cvg
Q Tbn And9gcqp3dqdaf2aefaimgggp8yf8qatgf5qmviyc91qoqyettnb2ijs Usqp Cau

Mourne Training Services System Suitability Failures
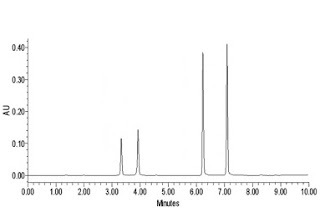
System Suitability In Hplc Analysis Pharmaceutical Guidelines

Clarity Hplc Data System System Suitability Test Extension
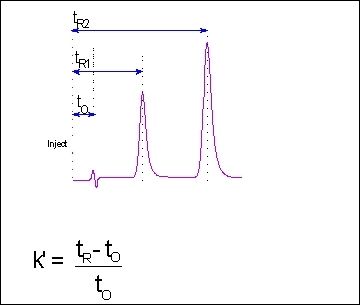
Dada Science Equipment Hplc System Suitability Test 5

Reverse Phase Hplc And Derivative Spectrophotometric Methods For Simultaneous Estimation Of Fenbendazole And Niclosamide In Pharmaceutical Dosage Form

A Validated Stability Indicating Rp Hplc Method Development And Validation For Simultaneous Estimation Of Cefixime Trihydrate And Levofloxacin Hemihydrate In Pharmaceutical Dosage Form
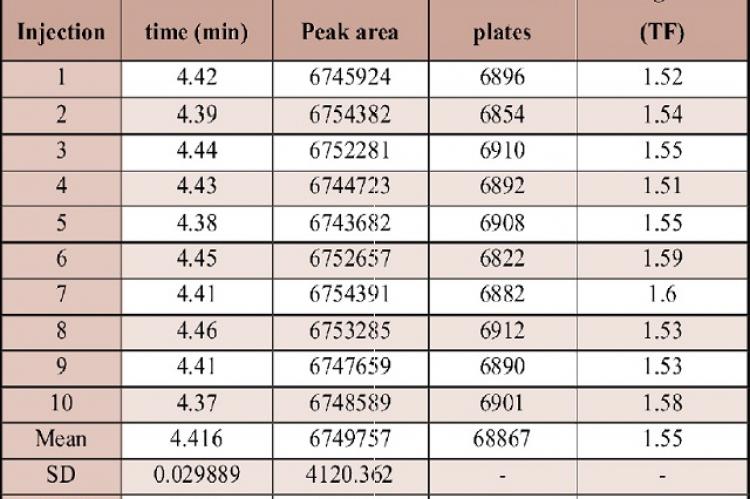
Analytical Method Development And Validation For The Estimation Of Cinnarizine By Rp Hplc In Bulk And Pharmaceutical Dosage Forms Asian Journal Of Pharmaceutical And Health Sciences

Development And Application Of A Validated Hplc Method For The Determination Of Clindamycin Palmitate Hydrochloride In Marketed Drug Products An Optimization Of The Current Usp Methodology For Assay
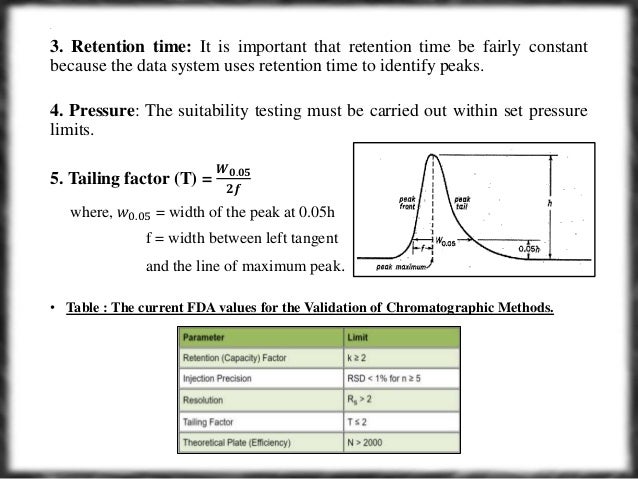
System Suitability Parameters Assessment By Hplc
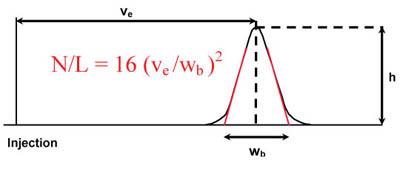
System Suitability Calculations Www Separations Eu Tosohbioscience Com

System Suitability Parameters Recommended For Hplc In Covalidation And Download Scientific Diagram
Current Validation Process And Issues Exemplified By The

Calculation Of System Suitability In Chromatography

מאמר סקירה באנגלית על התפקיד של כרומטוגרפיה נוזלית בלחץ גבוה בתעשיה הרוקחית פרמצבטית Shula Lc
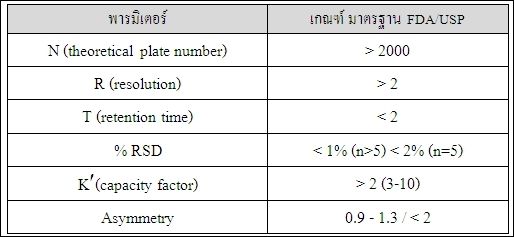
Dada Science Equipment Hplc System Suitability Test 6
2

Getting The Peaks Perfect System Suitability For Hplc

System Suitability Test Results For The Optimised Hplc Method For Download Table
Assets Thermofisher Com Tfs Assets Cmd Technical Notes Tn 708 Cds Automate System Suitability Testing Tn En Pdf
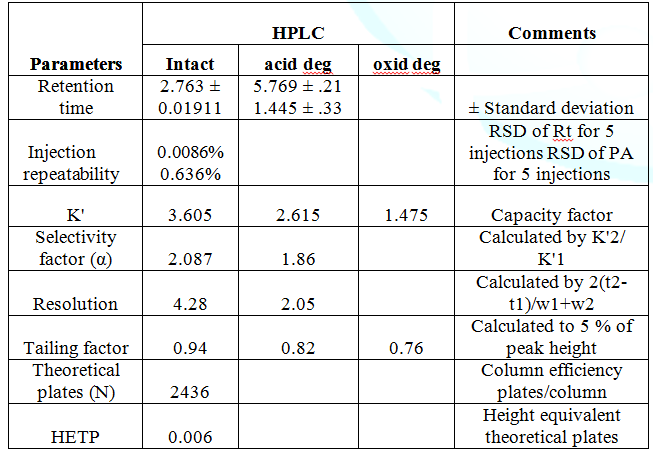
Bimatoprost Stress Stability Studies Edelweiss Pharma Analytic Acta

Data Integrity And Usp Part 3 Monitoring And Requalification Chromatography Online

System Suitability Parameters Of Rp Hplc For Tablet Analysis Download Table

Acid Alkali Degradation Study On Olmesartan Medoxomil And Development Of Validated Stability Indicating Chromatographic Methods
Ubidecarenone For System Suitability European Pharmacopoeia Ep Reference Standard 303 98 0 Sigma Aldrich

Uhplc Resolution System Suitability Specification Revision Chromatography Forum

Calculation Of System Suitability In Chromatography
.jpg)
Hplc System Stability
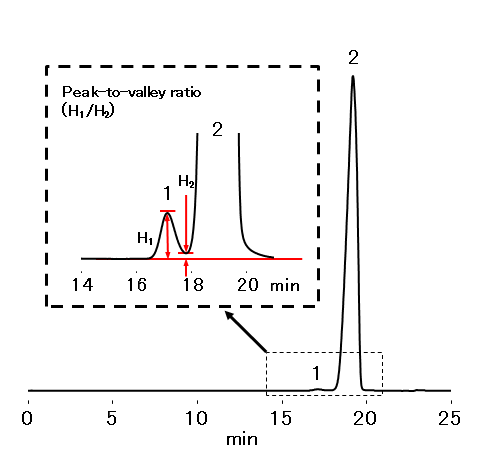
Analysis Of High Molecular Impurities In Insulin Aspart According To Pharmacopoeia Method Kw 802 5 Shodexhplc Com
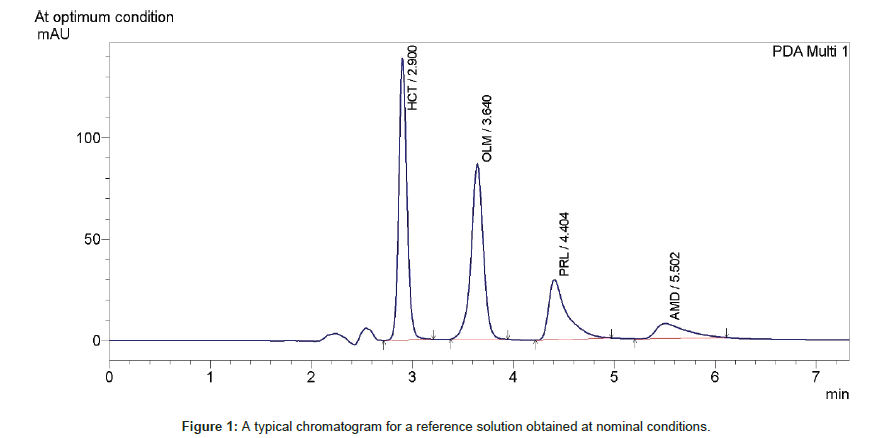
Definition Of System Suitability Test Limits On The Basis Of Robustness Test Results Omics International

Pharmapur Interview Questions On System Suitability Facebook

System Suitability Test Requirements In Chromatography

Dataapex Products Extensions Sst Extension
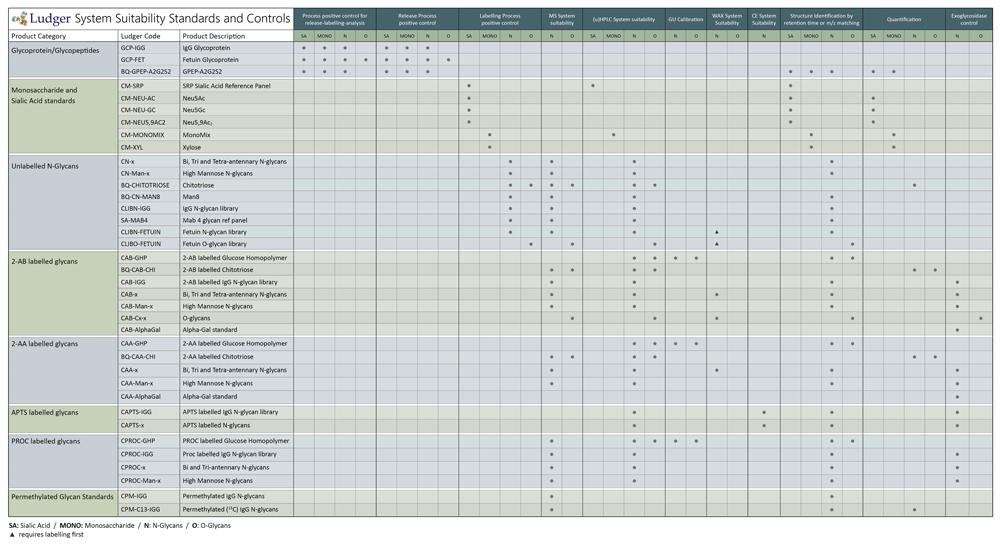
Hplc System Suitability Standards
Www Perkinelmer Com Lab Solutions Resources Docs Wtp Whysstisnosubstituteforaiq Pdf

Statistical Analysis Of Parameters Required For System Suitability Download Table

Figure 4 From Types Of Hplc Reversed Phase Chromatography Hplc Theory System Suitability Parameters The Role Of Hplc In Drug Analysis Semantic Scholar

Top Pdf System Suitability 1library
Www Bioglobax Com Wp Content Uploads 18 08 621 Chromatography Pdf

Definition Drug Contains Nlt And Nmt Of
Http Austinpublishinggroup Com Chromatography Fulltext Chromatography V1 Id1008 Pdf

Arc Hplc System Waters

Hplc Grade Acetonitrile Vwr International Bioz Ratings For Life Science Research

Acid Alkali Degradation Study On Olmesartan Medoxomil And Development Of Validated Stability Indicating Chromatographic Methods

Statistical Analysis Of The Parameters Required For System Suitability Download Table
Hplc
Www Rjpbcs Com Pdf 12 3 4 47 Pdf
Chromarogaphy System Suitability Ppt
Http Austinpublishinggroup Com Chromatography Fulltext Chromatography V1 Id1008 Pdf

Review Of Analytical Methods Ppt Video Online Download

Results Of System Suitability Tests Of Hplc Method Download Table

What Are System Suitability Tests Sst Of Analytical Methods

Why System Suitability Tests Are Not A Substitute For Analytical Instrument Qualification Pq Part 3 Calibration High Performance Liquid Chromatography
Hplc
Http Parasshah Weebly Com Uploads 9 1 3 5 Hplc Validation Pe Pdf

Mourne Training Services System Suitability Failures

Hplc Calibration Process Parameters In Terms Of System Suitability Test Semantic Scholar
Efficient Validated Method Of Hplc To Determine Amlodipine In Combinated Dosage Form Containing Amlodipine Enalapril And Bisoprolol And In Vitro Dissolution Studies With In Vitro In Vivo Correlation
Assets Thermofisher Com Tfs Assets Cmd Technical Notes Tn 723 Cds Automated System Suitability Testing Tn723 En Pdf
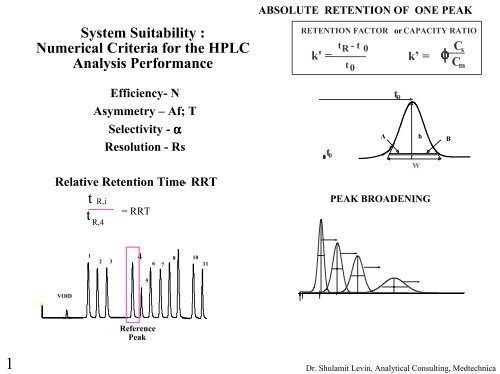
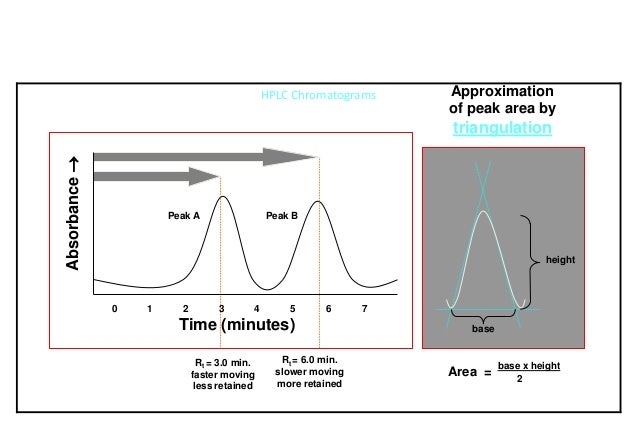
System Suitability Numerical Criteria For The Hplc Analysis
Www Edqm Eu Sites Default Files Presentation Pheur Training Ur Impurity Control May18 Pdf
Hplc
Kiran S Diary Interview Questions On System Suitability Parameters Hplc Gc

System Suitability Parameter Of Hplc Tailing Factor Retention Time Resolution Youtube
Innovareacademics In Journals Index Php Ajpcr Article Download 5344

Top Pdf System Suitability 1library
Chromarogaphy System Suitability Ppt
Research Birmingham Ac Uk Portal Files Broadhurst Et Al Guidelines Considerations Metabolomics Pdf

An Lc Ms Peptide Standard For Rapid System Suitability Assessment And Retention Time Translation Across Lc Ms Platforms Sigma Aldrich

Development And Validation Of Rp Hplc Method For The Pages 1 6 Flip Pdf Download Fliphtml5

System Suitability Calculations Www Separations Eu Tosohbioscience Com
Www Rjpbcs Com Pdf 12 3 4 47 Pdf

System Suitability In Bioanalytical Lc Ms Ms Sciencedirect
2

Usp Analysis Of Glimepiride On An Alliance Hplc System Modernization Of A Usp Method Waters
General Chapters 621 Chromatography System Suitability
12 Regulatory Aspects Of Hplc Analysis System Suitability 2 Mohsin Al Saleh
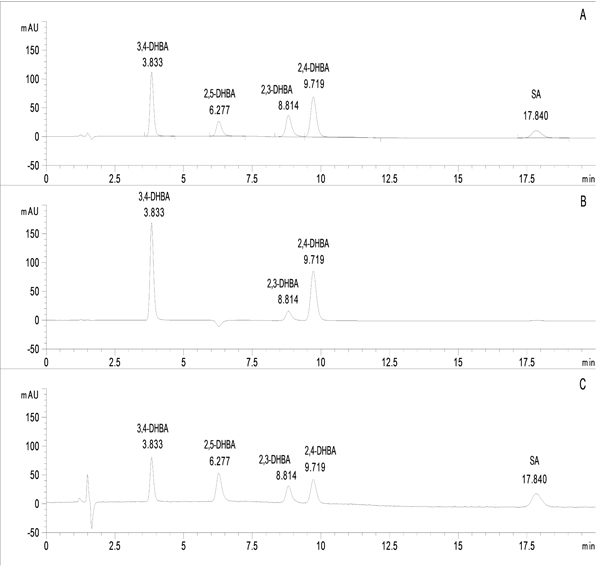
Oxidation Of Hydroxy And Dihydroxybenzoic Acids Under The Udenfriend S Conditions An Hplc Study

Calculation Of System Suitability In Chromatography



