Hplc System Suitability Parameters

Mourne Training Services System Suitability Failures

Data Integrity In The Gxp Chromatography Laboratory Part Iii Integration And Interpretation Of Data Chromatography Online
Assets Thermofisher Com Tfs Assets Cmd Technical Notes Tn 708 Cds Automate System Suitability Testing Tn En Pdf

System Suitability Parameters Recommended For Hplc In Covalidation And Download Scientific Diagram

J System Suitability Specifications And Tests High Performance Liquid Chromatography Elution

Calculation Of System Suitability In Chromatography
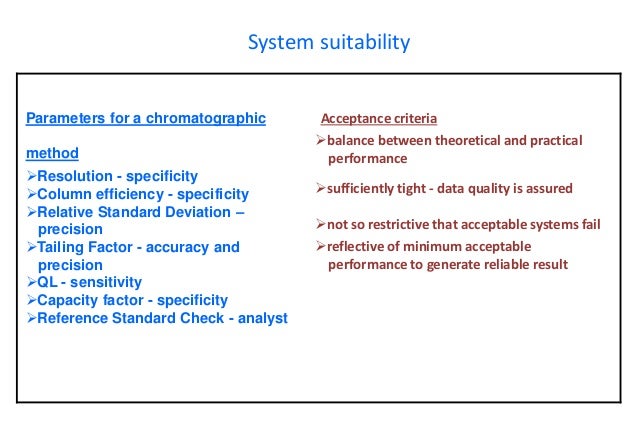
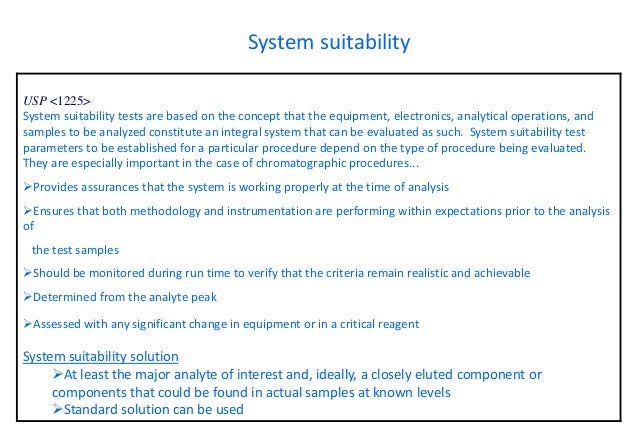
System suitability parameters should be selected during method validation Successful system suitability test runs ensure that the complete system meets the analyst's expectations under the specific conditions of the testsThe highest level of testing is the analysis of quality control samples.

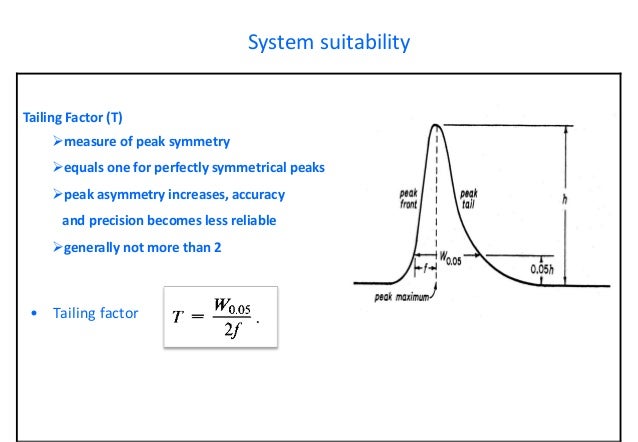
Hplc system suitability parameters. In this video, technical specialist Gordon Stack discusses system suitability and how this technique applies when measuring viscosity Using our patented VRO. T should be less than or equal to 2 to satisfy the system suitability requirement The tailing factor in HPLC is also known as the symmetry factor Precision Replicate injections of a standard preparation are used to ascertain if requirements of precision are met. System Suitability Calculations 1 Calculation of the number of Theoretical Plates per meter (USP method) 2 Calculation of the number of Theoretical Plates per meter (halfheight method) 3 Calculation of Peak Tailing (USP method) 4 Calculation of Peak Asymmetry 5 Calculation of the Height Equivalent to the Theoretical Plate (HETP) 6.
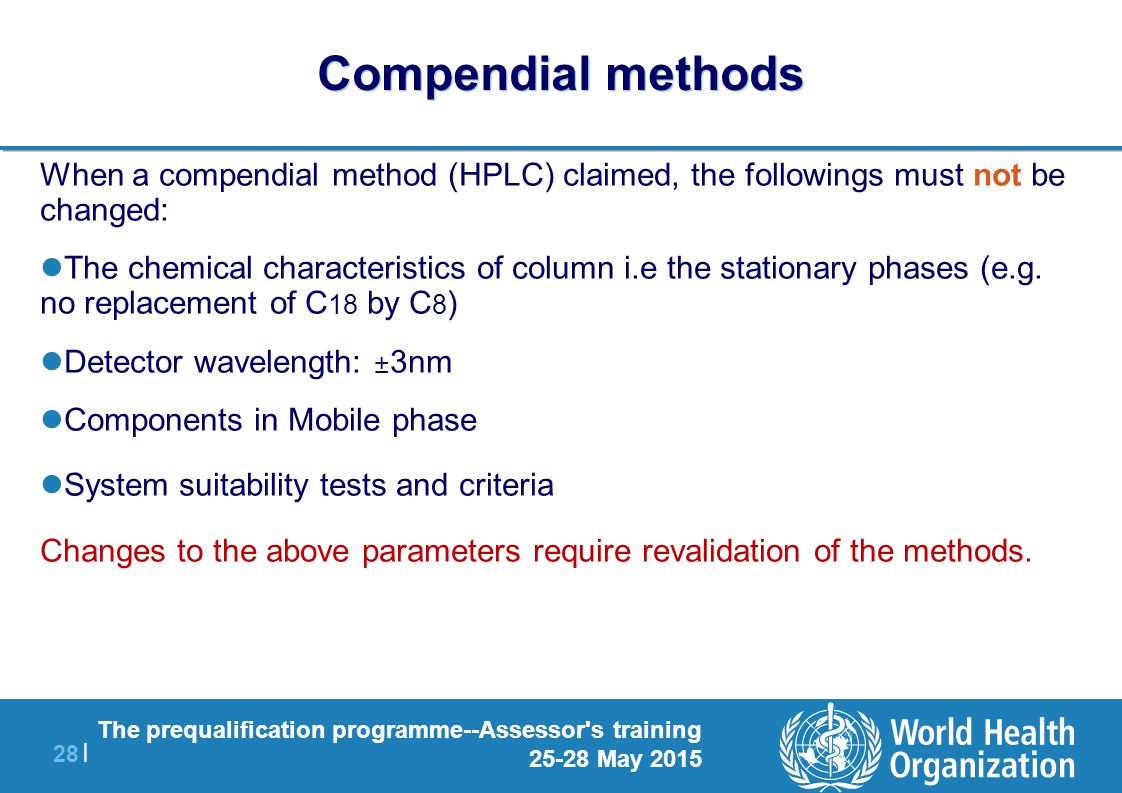
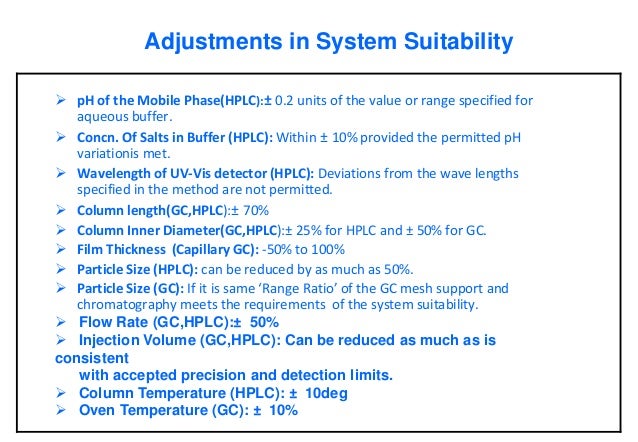
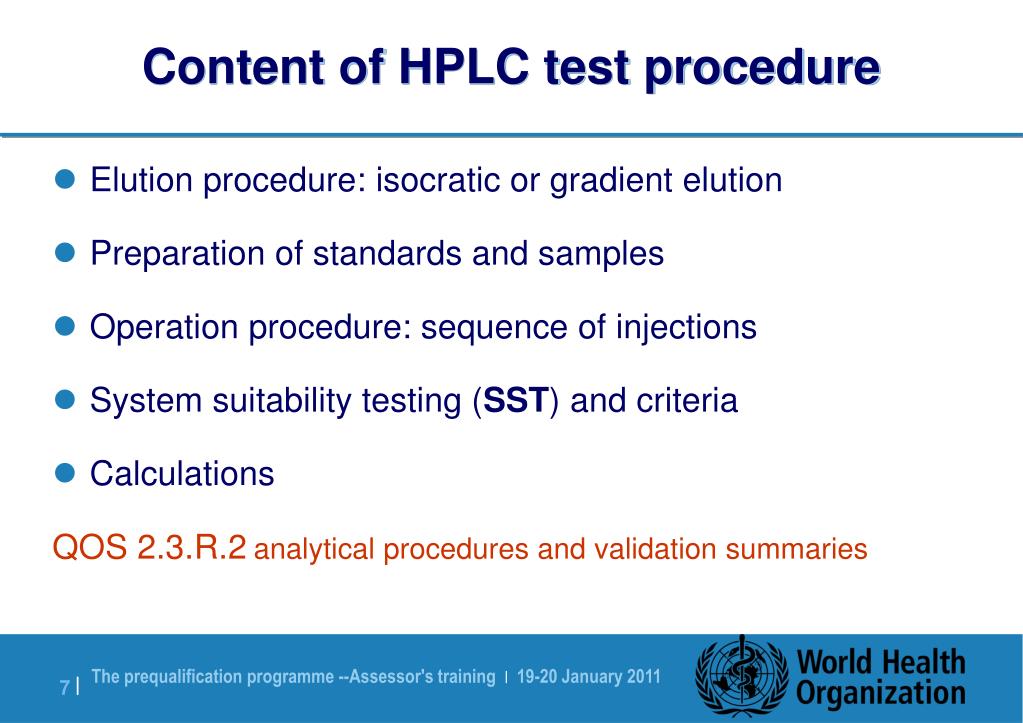
HPLC parameters such as column size, mobile phase condition etc if it is satisfying the requirements of system suitability Allowable adjustment parameters are described in General Chapter Chromatography of USP 41 and General Chapter 2246 Chromatographic separation techniques of EP 9 each Although both Pharmacopoeias permit to modify parameters, the allowable adjustments range may be different For example, it is possible to modify the. System suitability parameters System suitability parameters were analyzed to check the system performance consistency For system suitability parameters, six replicates of high quality control sample of TAP was injected, and column performances like tailing factor, retention time, and number of theoretical plates were observed (Table 1);. The injection sequence shall be in the following sequence to be use Blank → System suitability → Placebo (if required) → Standard → Sample → System suitability (at the end of analysis) The Standard shall be injecting in five replicate and sample is required to be inject in duplicate The system suitability (once established) shall be valid for a maximum period of 24 hours.
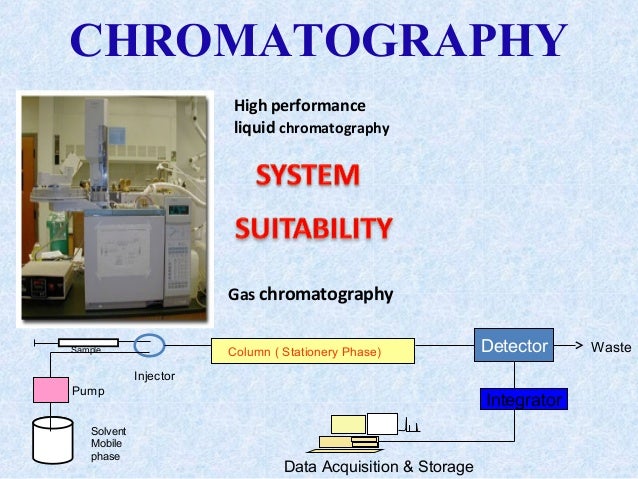
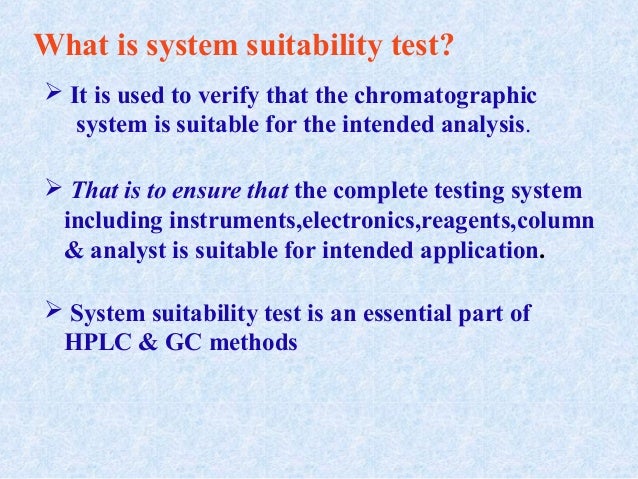
The system suitability test is used to verify that the chromatographic system is suitable for the intended analysis or not That is to ensure that the complete testing system including instruments, electronics, reagents, column & analyst is suitable for intended application The main system suitability parameters are 1 Precision 2 Capacity factor 3. The System Suitability Testing limits should conform to the guidelines provided by CDER (Center for Drug Evaluation and Research) Other sources for referencing about the System Suitability Testing are the USP (United States Pharmacopeia) and the ICH (The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use). HPLC Basics – Principles and parameters For HPLC system 05 mL/min and 50 mL/min or maximum flow rate used For UHPLC system 02 mL/min and mL/min or maximum flow rate us.
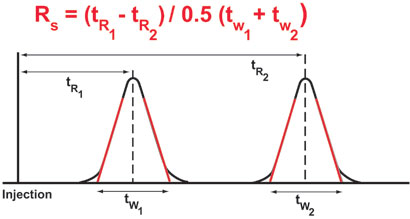
It is used to verify that the chromatographic system is suitable for the intended analysis That is to ensure that the complete testing system including instruments,electronics,reagents,column & analyst is suitable for intended application System suitability test is an essential part of HPLC & GC methods. System suitability parameters shall be checked by the analyst before proceeding with the sample analysis All solutions shall be clear homogeneous and free from particulate matter Filter the solutions before use While going through change over from reverse phase to normal phase and normal phase to reverse phase follow the changeover steps. Primary SST parameters are resolution (R), repeatability (RSD—relative standard deviations—of peak response and retention time), column efficiency (N), and tailing factor (T) These parameters are most important as they indicate system specificity, precision, and column stability.
A High Performance Liquid Chromatography (HPLC) HPL chromatographic separation is based on interaction and differential partition of the sample between the mobile liquid phase and the stationary. System suitability The HPLC system was equilibrated with the initial mobile phase composition, followed by 6 injections of the same standard HPLC Method Parameters That Can Be Varied System • Flow Rate / 50% • Injection Volume Increase up to 2x – maintain peak shape, resolution, retention time, etc tR2&tR1 are retention times of. System Suitability Calculations 1 Calculation of the number of Theoretical Plates per meter (USP method) 2 Calculation of the number of Theoretical Plates per meter (halfheight method) 3 Calculation of Peak Tailing (USP method) 4 Calculation of Peak Asymmetry 5 Calculation of the Height Equivalent to the Theoretical Plate (HETP) 6.
The following equations are related to System Suitability Please click on the corresponding tab below for the equations and details of how to calculate each one Dimensions when using HPLC or UHPLC columns, H is usually expressed in µm Calculation of Reduced Plate Height (h) Giddings introduced dimensionless parameters for H and also for. System Suitability Test The third layer of the data quality triangle is the system suitability test Again the basis for a SST working reliably is that the instrument is qualified and the method used is validated USP defines this as “Verify that the system will perform in accordance with the criteria set forth in the procedure”. 56 Allow the system to be saturated with mobile phase for at least 15 minutes before injecting the test sample 57 Record the area and retention time of both Benzene and Toluene in methanol 58 Calculate the system suitability parameters with the help of software such as resolution, tailing factor and theoretical plate.
Citation Bose A HPLC Calibration Process Parameters in Terms of System Suitability Test, Journal of chromatography System Suitability testing provides a means of checking that an entire chromatographic system is working within acceptable limits System suitability testing is an integral part of many analytical procedures Getting the peaks perfect System suitability for HPLC Online. System Suitability In addition, prior to the start of laboratory studies to demonstrate method validity, some type of system suitability must be done to demonstrate that the analytical system is performing properly Examples include • replicate injections of a standard preparation for HPLC and GC methods;. Chromatographic analytical results can be affected by various factors associated with the chromatographic system Requirements for system suitability testing (SST) are introduced to minimize the risk of such factors having a critical influence on the results This review covers the chromatographic parameters for SST and their recommended values and categorizes them into five groups depending.
For example, I have a system suitability injection to determine the. Some of the parameters which can be checked as SST requirements are Capacity Factor;. Table 2 shows the results for the system suitability parameters All parameters studied meet the acceptance criteria determined by FDA , demonstrating the repeatability of the method (%RSD ≤ 1), good peak symmetry (A s < 2), excellent column efficiency (N > 00), and wellresolved peaks from void volume (k’ > 2) Therefore, the quality performance of the chromatographic system was ensured to further validate the proposed RPHPLC method.
System suitability Up on injecting 100% level concentration, the data obtained from chromatograms illustrated that system suitability parameters include % RSD (≤ 2), USP tailing factor (≤ 2), and USP plate count (> 00) values shown in Table 2 were satisfying the acceptance criteria as per Q2 specifications of ICH guidelines. The below mentioned parameters are required to be complies during validation of HPLC method for Assay test 10 Specificity Demonstrate the separation of the analyte from Placebo Conduct the following forced degradation studies to obtain degraded sample, preferably 10 – 50% degradation and demonstrate the separation of the analyte from degradants. The simplest form of an HPLC system suitability test involves a comparison of the chromatogram trace with a standard trace (as shown below) This allows a comparison of the peak shape, peak width, baseline resolution Alternatively these parameters can be calculated experimentally to provide a quantitative.
Chapter 1 describes the System Suitability software and its place in an HPLC system Chapter 2 describes how to install the System Suitability software and how to load the contents of the project included on the System Suitability disk Chapter 3 describes the equations that Empower software uses to determine system suitability Related. In this I have explained briefly about all the system suitability parameter of HPLC analysis 1) % Related standard deviation (%RSD)/repeatability 2) Theoretical plate/Column efficiency 3. Does the system suitability parameters in a HPLC run requires to be met for all the injections throughout the sequence ?.
Incase of Inhouse product/ material if system suitability parameters ( theoretical plates, resolution, and tailing, etc) do not comply as per acceptance criteria but peak shape or peak elution pattern is good then send all relevant data to the analytical method development team for to review and revise the system suitability acceptance criteria. SST is an integral procedure to be done in every drug product analysis (qualitative or quantitative) USP(621) Chromatography gives the requirements for SST and acceptance criteria, unless it is. Signal to Noise ratio;.
The aim of the current study was the investigation of HPLC behaviour, separation and system suitability for the combination of Galantamine hydrobromide/Pymadine in model mixtures, in accordance with the new trend of multitarget therapy of Alzheimer’s disease by combining acetylcholinesterase inhibitor with its potential synergist. Hydrochloride Method Parameters Column 46 x 75 mm, 35 mm, StableBond SBCN (L10)1 Mobile Phase 55% 25 mM ammonium acetate pH 45/ 05% TEA 45% Acetonitrile 2 Flow Rate 1mL/min Detection UV 265 nm2 Temperature RT System Suitability Benzophenone and Diphenhydramine Solution Specifications Rs > , Tf < 25 for diphenhydramine. 1) WHAT IS SYSTEM SUITABILITY TEST?.
Hydrochloride Method Parameters Column 46 x 75 mm, 35 mm, StableBond SBCN (L10)1 Mobile Phase 55% 25 mM ammonium acetate pH 45/ 05% TEA 45% Acetonitrile 2 Flow Rate 1mL/min Detection UV 265 nm2 Temperature RT System Suitability Benzophenone and Diphenhydramine Solution Specifications Rs > , Tf < 25 for diphenhydramine. Normally with SST parameters we understand what is required by USP, EP,BP,JP,DAB, that is %RSD2% (6 injections), retention factor>2,tailing factor1. HPLC parameters such as column size, mobile phase condition etc if it is satisfying the requirements of system suitability Allowable adjustment parameters are described in General Chapter Chromatography of USP 41 and General Chapter 2246 Chromatographic separation techniques of EP 9 each Although both Pharmacopoeias permit to modify.
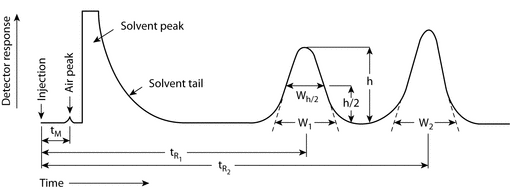
What is system suitability test?. System Suitability Parameters The system suitability parameters which are generally accepted by regulatory authorities and independent auditor are depicted below • Peak retention time, • Peak area, • Amount, • Peak height, • Peak width at half height, • Peak symmetry, • Peak tailing, • Capacity factor (k´), • Plate numbers, •. Where N = Number of theoretical plates V e = elution volume or retention time (mL, sec, or cm) h = peak height w 1/2 = width of the peak at half peak height (mL, sec, or cm) Calculation of the number of Theoretical Plates (USP method) Where N = Number of theoretical plates.
Before starting the analysis trial run to be carried out is required to check the Baseline noise, RT, system suitability parameters etc On completion, flush the system with lab water for 15minutes followed by organic solvent used in mobile phase water (50 50). 53 The results of the different parameters which are included in system suitability should be within the limit as per the respective method of analysis 54 Get the system suitability and chromatographs mention all the detail in respective product data sheet Related Principle of HPLC Chromatography 60 Abbrevaitions. High performance liquid chromatography (HPLC) is a suitable method for the analysis of a wide range of application areas Here, we describe the principle of HPLC and introduce to the most important components in an HPLC system and the factors that determine the success of a measurement.
System suitability testing is an integral part of many analytical procedures The tests are based on the concept that the equipment, electronics, analytical operations and samples to be analyzed constitute an integral system that can be evaluated as such System suitability test parameters to. Deciding on system suitability should look to chromatographers both inside and outside your system That is, conformance of a separation to a set of parameters should ensure the quality of work done under the specified chromatographic conditions, but don’t drive yourself or your operators crazy with needless sample prep, calculation, or report. Values of percentage of relative standard deviation.
Evaluating System Suitability • peak tailing, • capacity factor (k´), • plate numbers, • resolution between peaks, • selectivity relative to preceding peak, • skew, and • excess The mean value, the standard deviation, the relative standard deviation and the confidence interval are calculated You can set limits for either standard. Let’s look in to each of the System Suitability parameters. The system suitability (once established) shall be valid for a maximum period of 24 hours System suitability testing should be performed before the injection of samples No sample analysis is acceptable unless the requirements of system suitability have been met Sample analyses obtained while the system fails requirements are unacceptable.
System suitability tests are an integral part of gas and liquid chromatographic methods They are used to verify that the detection sensitivity, USP29 (Official June 1, 06) resolution, and reproducibility of the chromatographic system are adequate for the analysis to be done The tests are based on the concept that the equipment, electronics, analytical operations, and samples to be analyzed. In summary, system suitability testing failures can be reduced by a combination of three measures 1 Set system suitability criteria which relate specifically to the method in use A column degradation study will identify the parameters of resolution, tailing and efficiency which indicate that a new column should be used 2. Prepared as per procedure and was injected five times into the HPLC system The system suitability parameters were evaluated from standard chromatograms obtained by calculating the RSD of retention times, tailing factor, theoretical plates and peak areas from five replicate injections (Table 1)33.
System Suitability • Many calculations automatically performed – Tailing Factor – Resolution – Plate Count – Signal to Noise and EP Signal to Noise • Custom Calculations can be created. EXPLAIN SST PARAMETERS IN HPLC or GC?. The developed RPHPLC method was validated as per ICH guidelines The parameters validated are Specificity, Forced degradation studies, Accuracy, Precision (Intraday precision, Interday precision), Linearity, Limit of Detection (LOD), Limit of quantitation (LOQ), Solution stability, Robustness, and System suitability parameters.
A High Performance Liquid Chromatography (HPLC) HPL chromatographic separation is based on interaction and differential partition of the sample between the mobile liquid phase and the stationary. Chromatographic analytical results can be affected by various factors associated with the chromatographic system Requirements for system suitability testing (SST) are introduced to minimize the risk of such factors having a critical influence on the results This review covers the chromatographic parameters for SST and their recommended values and categorizes them into five groups depending. For this reasons SSTs are analysed before and during testing The most important SST parameters which are investigated for different HPLC analysis is Resolution (R), repeatability (RSD— relative standard deviations—of peak response and retention time), column efficiency (N), and Tailing factor (T).
Highpressure liquid chromatography (HPLC), sometimes called highperformance liquid chromatography, is a separation technique based on a solid stationary phase and a liquid mobile phase system suitability parameters are determined from the analyte peak System suitability must be demonstrated throughout the run by injection of an.

Calculation Of System Suitability In Chromatography

Pharmapur Interview Questions On System Suitability Facebook
Review Of Analytical Methods Ppt Video Online Download
Chromarogaphy System Suitability Ppt

System Suitability Parameters Of Rp Hplc For Tablet Analysis Download Table

New Technologies To Improve Hplc Compendial Pharmaceutical Methods
Kiran S Diary Interview Questions On System Suitability Parameters Hplc Gc
Www Agilent Com Cs Library Primers Public Best Practice Lc Operations Pdf
Www Pmda Go Jp Files Pdf
Www Separations Us Tosohbioscience Com
Chromarogaphy System Suitability Ppt

Review Of Analytical Methods Ppt Video Online Download

System Suitability Parameters Download Table
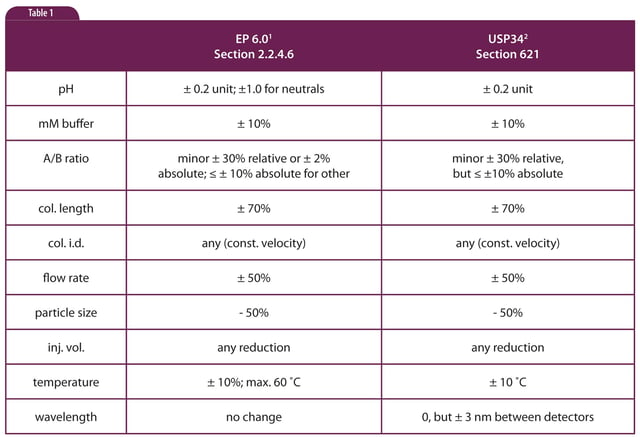
Method Adjustment Vs Change Part 1 Overview
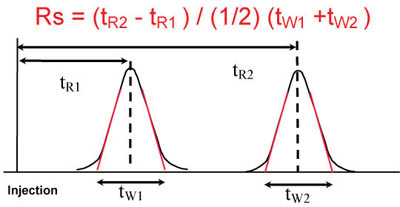
System Suitability Calculations Www Separations Eu Tosohbioscience Com

Pin On Hplc

Hplc Calibration Process Parameters In Terms Of System Suitability Test Semantic Scholar

Getting The Peaks Perfect System Suitability For Hplc
Www Bioglobax Com Wp Content Uploads 18 08 621 Chromatography Pdf
Q Tbn And9gcqp3dqdaf2aefaimgggp8yf8qatgf5qmviyc91qoqyettnb2ijs Usqp Cau

Q A On Hplc System Suitability Parameters Sst Parameters Pharmabeej Youtube

What Are System Suitability Tests Sst Of Analytical Methods

System Suitability Test Requirements In Chromatography

Quantitative Work In Hplc
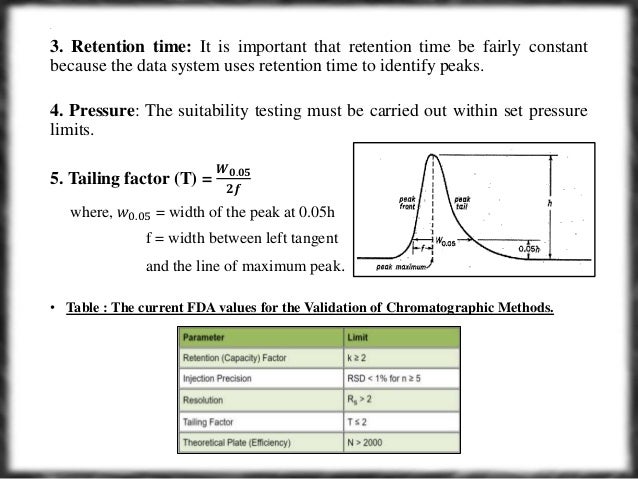
System Suitability Parameters Assessment By Hplc

J System Suitability Specifications And Tests High Performance Liquid Chromatography Elution

System Suitability Parameters Of Chromatogram For The Determination Of Download Table
Http Austinpublishinggroup Com Chromatography Fulltext Chromatography V1 Id1008 Pdf

Development And Validation For Simultaneous Estimation Of Budesonide And Salmeterol Xinafoate In Metered Dose Inhalation Form By Rp Hplc

A Validated Stability Indicating Rp Hplc Method Development And Validation For Simultaneous Estimation Of Cefixime Trihydrate And Levofloxacin Hemihydrate In Pharmaceutical Dosage Form

System Suitability Parameters For Dec And Cpm By Rp Hplc Method Download Table
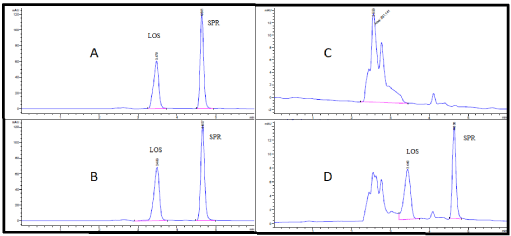
Developing A Highly Validated And Sensitive Hplc Method For Simultaneous Estimation Of Losartan And Spironolactone In Tablets And Human Plasma

Hplc Calibration Process Parameters In Terms Of System Suitability Test Semantic Scholar

Mourne Training Services System Suitability Failures
Http Parasshah Weebly Com Uploads 9 1 3 5 Hplc Validation Pe Pdf

מאמר סקירה באנגלית על התפקיד של כרומטוגרפיה נוזלית בלחץ גבוה בתעשיה הרוקחית פרמצבטית Shula Lc

Review Of Analytical Methods Ppt Video Online Download
Hplc
Definition Of System Suitability Test Limits On The Basis Of Robustness Test Results Omics International

Reverse Phase Hplc And Derivative Spectrophotometric Methods For Simultaneous Estimation Of Fenbendazole And Niclosamide In Pharmaceutical Dosage Form
Hplc

System Suitability Numerical Criteria For The Hplc Analysis

System Suitability Parameters Of Hplc Resolution Retention Time Tailing System Suitability Youtube
Mohsinalsaleh Com Wp Content Uploads 17 03 12 Regulatory Aspects Of Hplc Analysis System Suitability 2 Pdf

Q A On Hplc System Suitability Parameters Sst Parameters Pharmabeej Youtube
621 Chromatography

Usp Analysis Of Glimepiride On An Alliance Hplc System Modernization Of A Usp Method Waters
Mohsinalsaleh Com Wp Content Uploads 17 03 12 Regulatory Aspects Of Hplc Analysis System Suitability 2 Pdf
Definition Of System Suitability Test Limits On The Basis Of Robustness Test Results Omics International

Method Validation And Verification Protocols For Test Methods Online Presentation
Hplc

Development And Application Of A Validated Hplc Method For The Determination Of Clindamycin Palmitate Hydrochloride In Marketed Drug Products An Optimization Of The Current Usp Methodology For Assay

Development And Validation Of New Rp Hplc Method For The Estimation Of Alfuzosin Hydrochloride In Bulk And Tablet Dosage Form
Www Mdpi Com 79 63 8 4 268 Pdf
Http Austinpublishinggroup Com Chromatography Fulltext Chromatography V1 Id1008 Pdf

Mourne Training Services System Suitability Failures
Hplc

Hplc Calibration Process Parameters In Terms Of System Suitability Test Semantic Scholar

An Lc Ms Peptide Standard For Rapid System Suitability Assessment And Retention Time Translation Across Lc Ms Platforms Sigma Aldrich

Development And Validation Of Rp Hplc Method For The Pages 1 6 Flip Pdf Download Fliphtml5

Usp Analysis Of Glimepiride On An Alliance Hplc System Modernization Of A Usp Method Waters
Ppt Method And Validation Basics Hplc Case Study Hua Yin Assessor Powerpoint Presentation Id

Pharmapur Interview Questions On System Suitability Facebook

Multivariate Optimization For Simultaneous Determination Of Aspirin And Simvastatin By Reverse Phase Liquid Chromatographic Method Using Aqbd Approach Sciencedirect

System Suitability In Hplc Analysis Pharmaceutical Guidelines

Calculation Of System Suitability In Chromatography

Hplc Calibration Process Parameters In Terms Of System Suitability Test
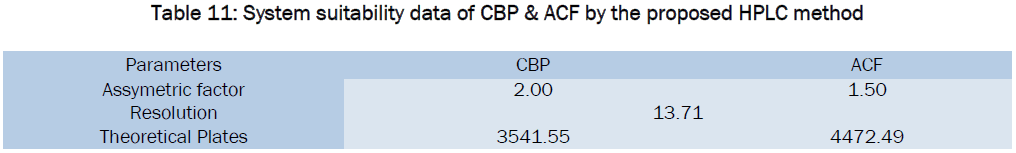
Liquid Chromatographic Estimation Of Cyclobenzaprine Hydrochloride And Aceclofenac In Pharmaceutical Formulation Open Access Journals
Http Informaticsjournals Com Index Php Ajprhc Article Download 639 605
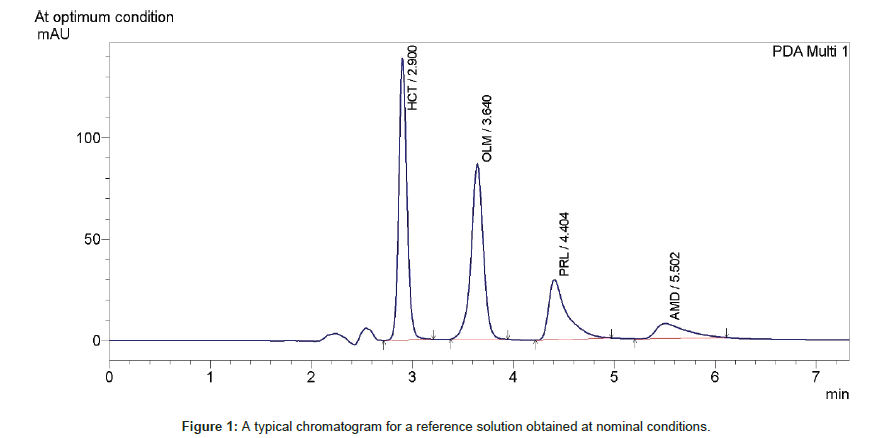
Definition Of System Suitability Test Limits On The Basis Of Robustness Test Results Omics International

Acceptance Criteria For System Suitability Parameters Download Scientific Diagram
Assets Thermofisher Com Tfs Assets Cmd Technical Notes Tn 708 Cds Automate System Suitability Testing Tn En Pdf

Dataapex Products Extensions Sst Extension
System Suitability Parameters Of Hplc Resolution Retention Time Tailing System Suitability Youtube
Http Parasshah Weebly Com Uploads 9 1 3 5 Hplc Validation Pe Pdf

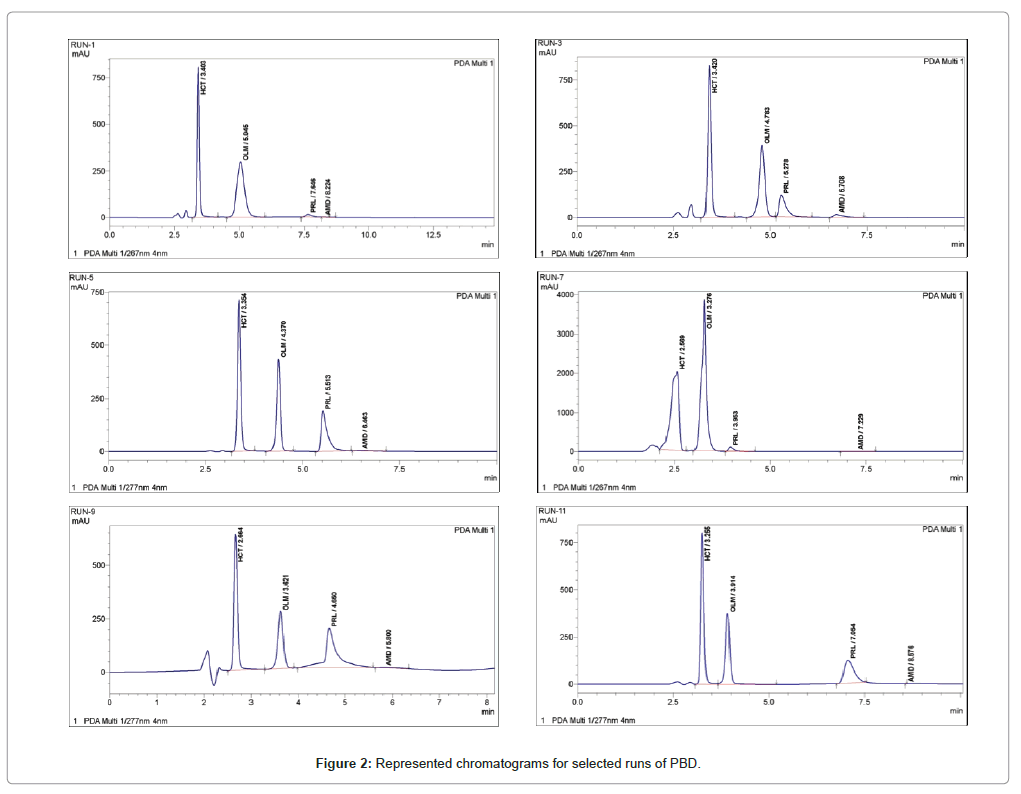
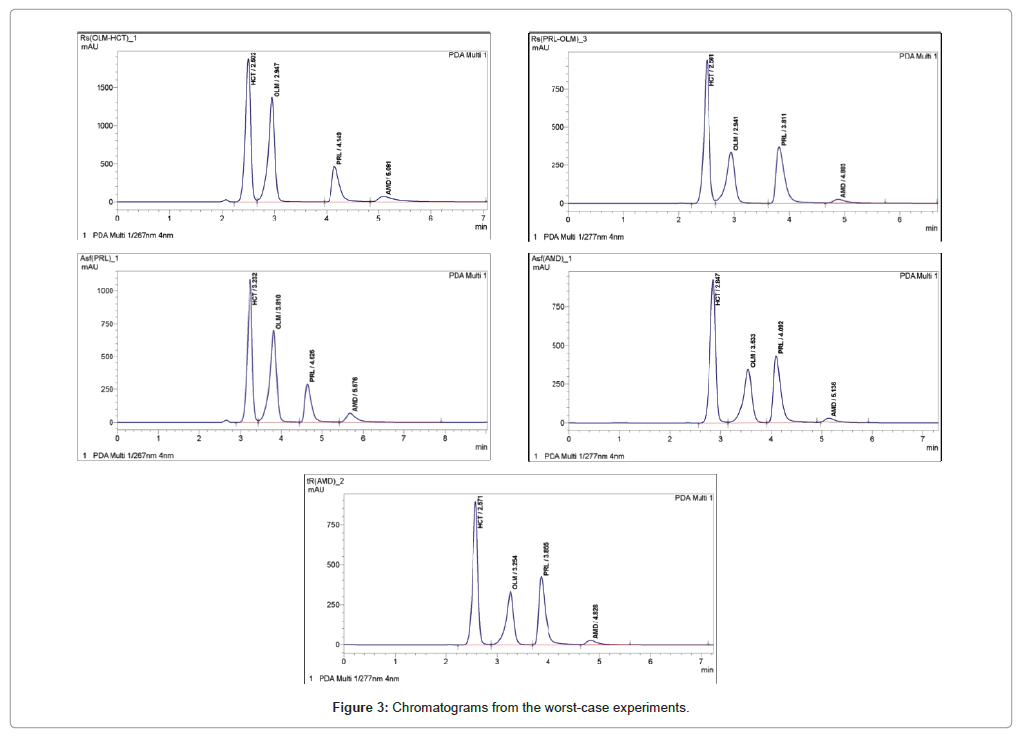
Acid Alkali Degradation Study On Olmesartan Medoxomil And Development Of Validated Stability Indicating Chromatographic Methods
Www Bioglobax Com Wp Content Uploads 18 08 621 Chromatography Pdf
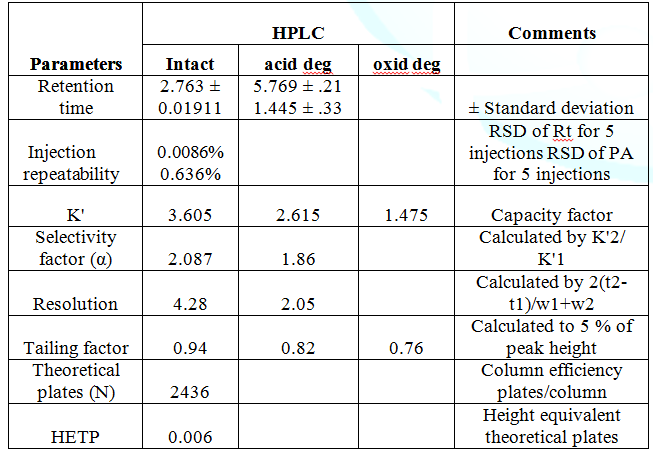
Bimatoprost Stress Stability Studies Edelweiss Pharma Analytic Acta

Acid Alkali Degradation Study On Olmesartan Medoxomil And Development Of Validated Stability Indicating Chromatographic Methods
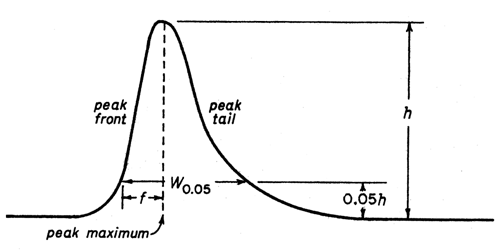
General Chapters 621 Chromatography System Suitability

System Suitability Test Requirements In Chromatography
Introduction Springerlink

Kiran S Diary Interview Questions On System Suitability Parameters Hplc Gc

Results Of System Suitability Tests Of Hplc Method Download Table
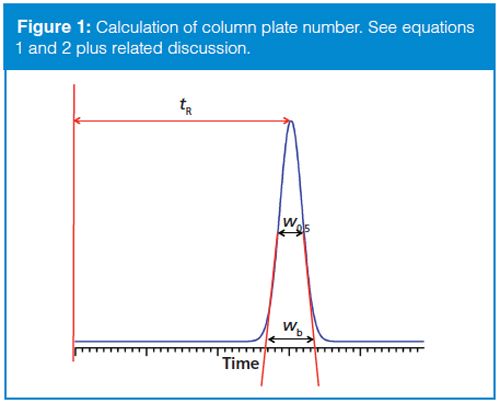
Column Plate Number And System Suitability Chromatography Online
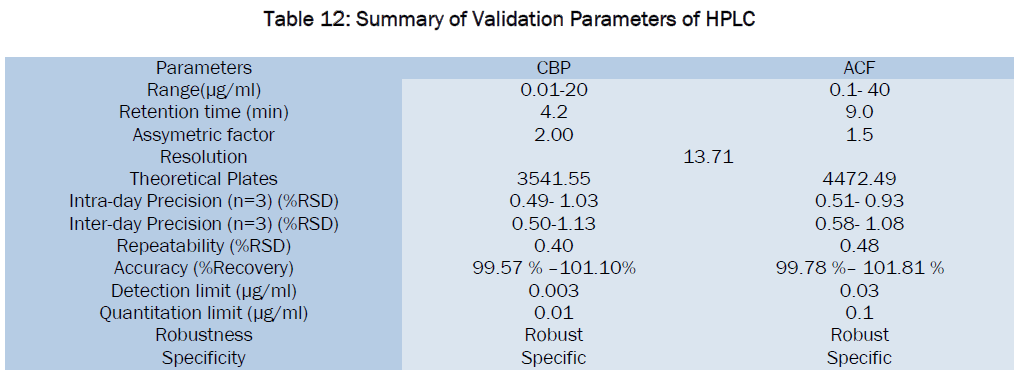
Liquid Chromatographic Estimation Of Cyclobenzaprine Hydrochloride And Aceclofenac In Pharmaceutical Formulation Open Access Journals
2

Hplc System Suitability Parameters For 5 Asa Analysis Download Scientific Diagram

Revision Of European Pharmacopeia Ep Chapter 2 2 46
Www Rjpbcs Com Pdf 12 3 4 47 Pdf
2
Http Cms Galenos Com Tr Uploads Article 124 323 334 Pdf
System Suitability In Hplc Analysis Pharmaceutical Guidelines

Statistical Analysis Of Parameters Required For System Suitability Download Table
2



